InflammX Therapeutics, Inc.
Novel Upstream Technologies for Treating Diseases where the Inflammasome (NLRP3) Pathway of Inflammation has become dysregulated and autoinflammatory
Xiflam™
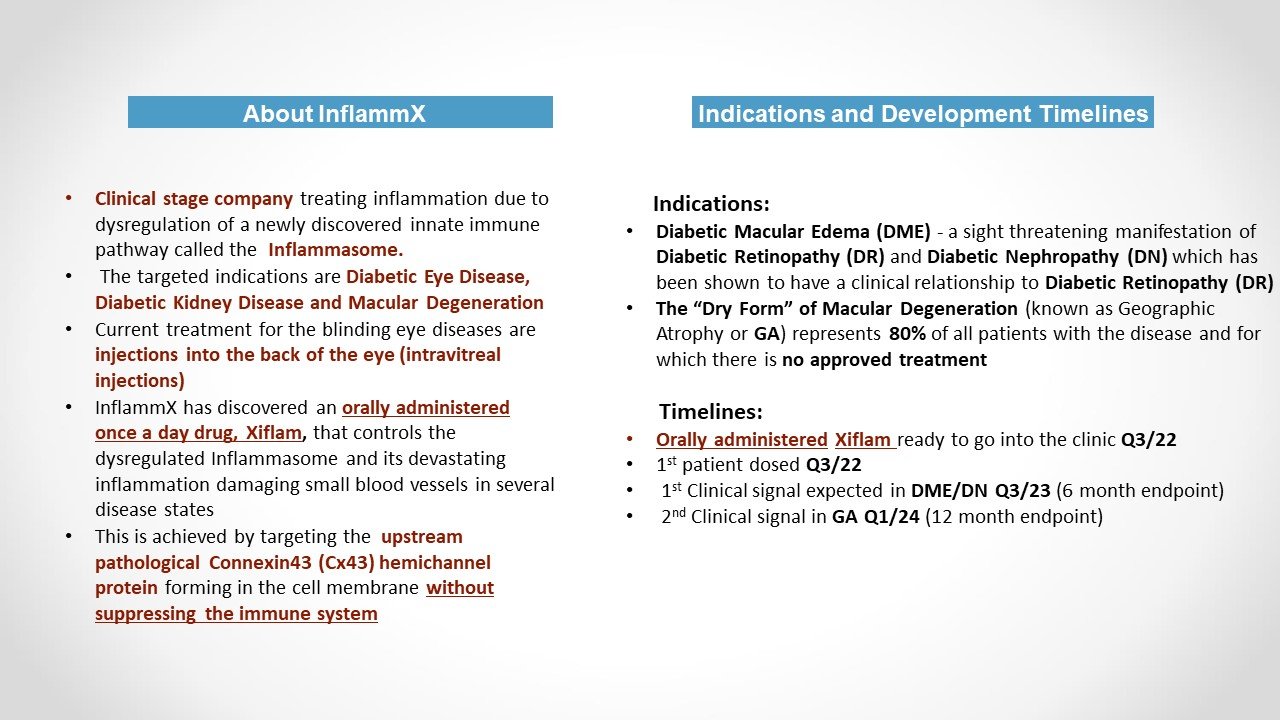
The lead product in Clinical Development, is Xiflam™, a once a day orally administered small molecule. The therapeutic target is the pathologically open Connexin43 (Cx43) Hemichannel. The pathological Cx43 hemichannel is responsible for inflammasome mediated autoinflammation in several disease states with significant unmet needs.
Current indications are Diabetic Macular Edema(DME)/Diabetic Chronic Kidney Disease (CKD) and the intermediate stage of Macular Degeneration (iAMD)
InflammX Therapeutics is a clinical-stage company with highly differentiated, first-in-class products based upon a novel mechanism called “Connexin43 (Cx43) Hemichannel Modulation.” This mechanism leads to a differentiated treatment paradigm which modifies the dysregulated (NLRP3) inflammasome pathway of inflammation and its downstream pathology. InflammX is developing Xiflam™, a small molecule for once a day oral administration. Xiflam™ has been shown to inhibit the pathologically open Cx43 hemichannel and modulate activation and dysregulation of the inflammasome resulting in perpetuated autoinflammation.
The initial indications for Xiflam™ are diseases in ophthalmology/nephrology with significant unmet need. Specifically these are diabetic retinopathy (DR), characterized by diabetic macular edema (DME), and diabetic nephropathy (DN) characterized by chronic kidney disease (CKD) and the intermediate (early stage) of AMD (iAMD).
iAMD is the precursor of the later stage of disease known as Geographic Atrophy (GA) which results in a significant loss of visiual function.
Currently there is no approved treatment for GA and those in development require monthly intravitreal (into the back of the eye) injections for life. For DR the treatment is monthly intravitreal injection of an anti Vascular Endothelial Growth Factor (VEGF) compound. Data to date suggests that the treatment is ineffective in approximately 50% of cases.
Since Xiflam™ is targeting inflammasome mediated and recycling inflammation, and providing a therapeutic effect to both eyes simultaneously (intravitreal injection can only be administered to one eye at a time), our data provides for the potential of significantly improving the treatment for these blinding disease.
Furthermore the clinical and scientific literature recognizes the correlations and similarities in the microvascular disease present in DR and CKD. Since diabetes related kidney disease is a significant morbidity in patients with diabetes and Xiflam™ will be orally dosed, the effect on kidney disease will be evaluated along with the retinal disease in the upcoming clinical trial. Additionally, the pathology in several neurodegenerative diseases have been shown to be mediated by the NLRP3 inflammasome dysregulation, resulting in autoinflammatory signs and symptomatology.